Sex-specific roles of purinergic signaling in the pathogenesis of inflammatory diseases
Project lead
| Prof. Qinghua Hu |
Professor of School of Life Science and Technology Dean of School of Life Science and Technology China Pharmaceutical University Email: huqh@cpu.edu.cn | |
| Prof. Hui Shen |
School of Life Science and Technology China Pharmaceutical University Email: shhui@cpu.edu.cn |
Graphic abstract
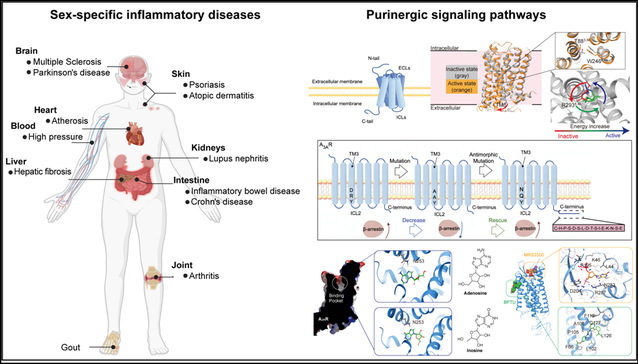
Inflammatory diseases often exhibit striking sex differences in incidence, clinical presentation, and immunopathology. Epidemiological studies have shown that certain inflammation-driven disorders occur more frequently in males, whereas others display a pronounced female bias and continue to rise in prevalence among women. These divergent patterns suggest that sex shapes disease susceptibility and immune reactivity through multifactorial mechanisms. Purinergic signaling is widely conserved across diverse species and plays essential regulatory roles in intercellular communication. Extracellular purine and pyrimidine nucleotides engage a variety of receptors to orchestrate coordinated cellular responses. These receptors include ionotropic P2X receptors and metabotropic P1 and P2Y receptors. In humans, P2Y receptors (P2YRs) responsive to nucleotides comprise eight members, which can be classified into two subfamilies based on G-protein coupling specificity. The P2Y1-like subfamily, which primarily couples to Gq/11 proteins (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R), and the P2Y12-like subfamily, which primarily couples to Gi/o proteins (P2Y12R, P2Y13R, and P2Y14R). Distinct P2Y receptors exhibit differential affinities for various extracellular nucleotides, contributing to the complexity and precision of purinergic signaling. These receptors are broadly expressed in immune cells and central nervous system glial cells and have been strongly implicated in regulating inflammatory and immune processes. Recent studies, including our own work, indicate that P2Y receptor-mediated pathways may contribute to sex-biased pathogenesis in multiple inflammatory disease contexts.
Sex differences in physiology and pathology have traditionally been attributed to disparities in sex hormone levels. However, emerging evidence highlights a critical contribution from sex chromosome-encoded proteins (Y-linked and X-linked factors), which can independently modulate immune function and disease outcomes. Deciphering how hormone-dependent and chromosome-encoded determinants give rise to sex-dimorphic phenotypes in inflammatory diseases will offer deeper insight into disease mechanisms and provide a foundation for precision medicine.
Building on our preliminary data demonstrating important roles for P2Y receptors in inflammatory pathogenesis, we plan to conduct broad, multidimensional analyses of peripheral blood and diseased tissues from male and female patients, as well as from corresponding animal models. Through transcriptomic profiling, immunohistochemistry, and flow cytometry, we will systematically characterize sex-specific expression patterns of P2Y receptor subtypes. These approaches will quantify receptor abundance, define spatial distribution, and correlate these parameters with clinical features, disease severity, and inflammatory mediators.
Sex-related differences in immune cell composition and functional states represent another major focus of our investigation. Previous studies have shown, for example, that females often exhibit higher proportions of CD4⁺ T cells and more robust Treg activity, whereas males may display enhanced neutrophil recruitment and amplified inflammatory cascades. Building on this knowledge, we will profile immune phenotypes and assess cellular functions to compare how immune cell subsets, downstream P2Y receptor signaling pathways, and pro- versus anti-inflammatory cytokine profiles differ between sexes. These analyses will help elucidate how immune architecture and purinergic signaling jointly shape sex-dependent susceptibility and disease severity.
By comparing inflammatory conditions that preferentially affect males with those more common in females, we aim to identify shared mechanistic themes underlying sex-specific immune regulation. Such mechanisms may arise from differential sensing of extracellular nucleotide signals, sex-specific metabolic states, or distinct hormonal microenvironments. Collectively, these factors may converge to produce sexually dimorphic immune responses across diverse inflammatory settings. This will deepen our understanding of the biological basis of sex differences in inflammatory diseases and provide new conceptual frameworks and molecular targets for individualized therapeutic strategies.
Team
Dr. Yafei Fang
Important publications
1. Fang Y, Wang J, Liu C, Yin L, Li Y, Ji C, Zhou M, Zhou M, Hu Q*. Targeting P2Y14R alleviates platelet-induced NET formation and venous thrombosis through PKA/AKAP13/RhoA axis. Eur Heart J (2025).
2. Zhou M, Li Y, Qian J, Dong X, Guo Y, Yin L, Liu C, Hao K, Hu Q*. P2Y14R activation facilitates liver regeneration via CREB/DNMT3b/Dact-2/ β -Catenin signals in acute liver failure. Acta Pharm Sin B (2025).
3. Li Y, Zhou M, Li H, Dai C, Yin L, Liu C, Li Y, Zhang E, Dong X, Ji H, Hu Q*. Macrophage P2Y6 receptor deletion attenuates atherosclerosis by limiting foam cell formation through phospholipase C β /store-operated calcium entry/calreticulin/scavenger receptor A pathways. Eur Heart J (2024).
4. Liu C, Wang H, Han L, Zhu Y, Ni S, Zhi J, Yang X, Zhi J, Sheng T, Li H, Hu Q*. Targeting P2Y14R protects against necroptosis of intestinal epithelial cells through PKA/CREB/RIPK1 axis in ulcerative colitis. Nat Commun (2024).
5. Yin L, Zhang E, Mao T, Zhu Y, Ni S, Li Y, Liu C, Fang Y, Ni K, Lu Y, Li H, Zhou M, Hu Q*. Macrophage P2Y6R activation aggravates psoriatic inflammation through IL-27- mediated Th1 responses. Acta Pharm Sin B (2024).
6. Wang YH, Zhou MZ, Ye T, Wang PP, Lu R, Wang YL, Liu CX, Xiao W, Li JY, Meng ZB, Xu LL, Hu Q*, Jiang C. Discovery of a Series of 5-Amide-1H-pyrazole-3-carboxyl Derivatives as Potent P2Y14R Antagonists with Anti-Inflammatory Characters. J Med Chem (2022).
7. Yin L, Ni K, Mao T, Tian S, Liu C, Chen J, Zhou M, Li H, Hu Q*. Attributes novel drug candidate: Constitutive GPCR signal bias mediated by purinergic receptors. Pharmacol Ther (2025).
8. Shen H*, Yanas A*, Owens MC, Zhang C, Fritsch C, Fare CM, Copley KE, Shorter J, Goldman YE, Liu KF. Sexually dimorphic RNA helicases DDX3X and DDX3Y differentially regulate RNA metabolism through phase separation. Mol Cell(2022).
9.Owens MC *, Shen H*, Yanas A, Mendoza-Figueroa MS, Lavorando E, Wei XY, Shweta H, Tang HY, Goldman YE,Liu KF. Specific catalytically impaired DDX3X mutants form sexually dimorphic hollow condensates.Nat Commun(2024).
10.Minxiang Zhong*, Shiyuan Chen*, Wengqi Lu, Ting Luo, Ruiya Shi, Jinzhou Li,Shen H. Liquid-liquid phase separation of DDX3X: mechanisms, pathological implications, and therapeutic potential.Int J Biol Macromol(2025).




